Serum free media - All are not created equal
Posted by Sudhirdas Prayaga, Ph.D. President and CEO, Antibody Research Corporation, St Charles, Missouri, USA on May 26th 2017
The pharmaceutical industry went through a major paradigm change in their product development strategy. Until recently they were very much focused on small molecule based drug development. Things started changing over last 10 years or so when more and more protein based biologics got approvals and some became blockbuster drugs. Probably the success of Rituxan/MabThera, for the treatment of Non Hodgkins lymphoma launched in 1997 by Idec Pharmaceuticals and Genentech, initiated this shift towards biologics. Also poor success rate recently with small molecules have contributed to this change. Although the genomic revolution was to bring more drugs to market faster it did fare well as hyped. Currently, monoclonal antibody development is the fastest growing segment in biotech industry recording 14% annual growth rate (versus 0.6% for small molecule), with recent recorded sales in excess of $20 billion. Over 50% of products in development are biologics that include monoclonal antibodies and recombinant proteins.
The manufacture of biologics requires live cells such as bacterial culture, yeast or mammalian cell culture. Many recombinant proteins and antibodies require mammalian cell culture for production. Cells are grown in vitro for the production of proteins they produce including antibodies. About half of all recombinant therapeutic proteins currently in the market are produced in mammalian cells. The accelerated demand of biologics also put lot of demand on bioprocessing to increase capacity, improve on efficiency and yield. Significant progress has been made in in vitro cell culture technologies during last few years. Initial commercial uses of cell culture were for the production of vaccines, such as polio vaccines.
Bovine serum has been used to supplement media for growth of animal cells. Serum provides over 1000 different components, including many essential nutrients, most of which are unknown. Although addition serum delivers many advantageous to cell culture, it also brings with it many important draw backs such as safety issues and batch to batch variability during manufacturing. Adventitious agents such as Bovine Spongiform Encephalopathies (BSE) and foot & mouth disease are a major safety concern during manufacturing of biologics. This has led to a drive to remove serum and products of animal origin form cell culture media during manufacture of protein-based biologics.
Initially serum free medium was introduced into biopharmaceutical manufacturing because of the cost and process constraints of serum. Ham first used serum free medium in mammalian cell culture in 1965. The classical basal media used in manufacturing such as RPMI, IMDM or DMEM are supplemented with 5-20% serum. Biopharmaceutical companies are now requiring cell culture media to be animal free, serum free, defined, and cost effective. Various formulations of media have been developed from reduced serum media to completely defined media. Serum free media (SFM) are basal media without serum, but supplemented with various proteins and extracts from other sources, may or may not of animal origin. Animal component free (ACF) media are media without serum or proteins of animal origin. Protein free media (PFM) are basal media without any proteins, but may still contain chemical of unknown chemical nature such as plant or yeast hydrolysates. Chemically Defined media (CDM) are basal media with additives only of biochemically-defined low molecular weight constituents.
Antibody Research Corporation is a contract research and manufacturing service provider to industry and academia. When we receive a new cell line for bio manufacturing we screen them with over 20 different serum free media from 9 vendors to determine best media for growth and productivity (see table-1). We see large variation in both growth and productivity for choice of media between cell lines. Some media support growth very well, but start loosing viability quickly once they reach confluency. While others support slow growth, but maintain viability once confluency is reached. Many vendors also provide dedicated media for commonly used cell lines such as CHO, HEK293 or hybridoma. Our experience is that it doesn’t always work like that. For example a CHO media may work as good or better than hybridoma media from the same vendor for a particular hybridoma cell line.
Table-1: Some Serum free or protein free media and their manufactures No. Media Vendor 1 CHO Medium BD 2 Cell MAB BD 3 HyQ SFM4CHO Hyclone 4 HyQ CDM4CHO Hyclone 5 HyQ ADCF-Mab Hyclone 6 CD CHO Medium Invitrogen 7 CD Hybridoma Invitrogen 8 CHO-S-SFM II Invitrogen 9 CHO AFS KC Bio 10 Liforcell Life Blood Medical 11 Prodoma 1 Lonza 12 Prodoma 2 Lonza 13 Prodoma 3 Lonza 14 Cellgro Free MediaTech 15 TurboDoma MediaTech 16 TP6 MediaTech 17 Lipumin PAA 18 Ex-Cell 302 CHO SAFC Biosciences 19 Ex-Cell Sp2/0 SAFC Biosciences 20 Ex-Cell 293 SAFC Biosciences
Our initial matrix screen is to identify best media for growth. Typically we see about 25% of media supporting good growth and productivity, 25% no growth at all. And about 50% lie in between these two extremes. The top 4 or 5 media chosen will be further analyzed for both growth and productivity (see figure 1a & b). The ideal media should support fast growth, but once reached confluency should stop dividing and produce the biopharmaceutical. Media can be changed either in a batch format or continuously after checking for level of glucose and lactate. Companies have also come up with media formulation one for growth and another for productivity when high cell density is reached. We are researching media additive that can be added to culture at appropriate cell densities that can drive cells from growth mode to productivity. These can be a simple agent that can prevent apoptosis or complex growth factors that induce differentiation.
Figure-1a Growth of a hybridoma cell line clone-1 in 5 serum free media. Growth in DMEM with 10% serum used as control. Cells were plated in 96 well plates at 100,000 cells/mL and cell density determined after 1, 2 or 3 days in culture.
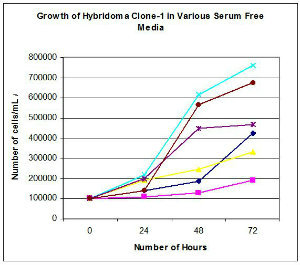
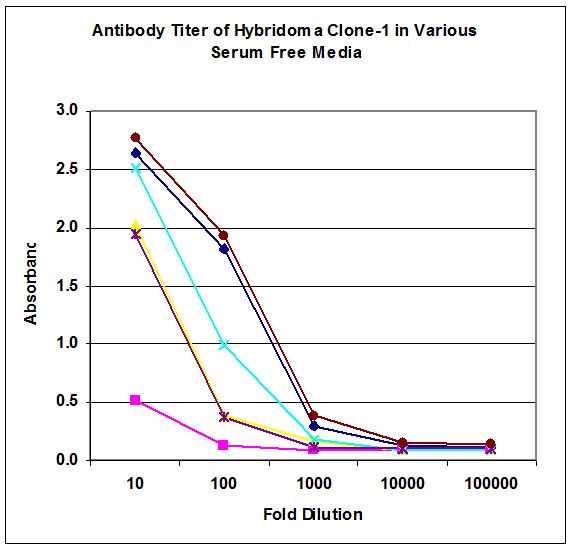
Figure-1b Productivity of hybridoma cell line clone-1 in 5 serum free media. Growth in DMEM with 10% serum used as control. Cells were plated in 96 well plates at 100,000 cells/mL and IgG titer determined by ELISA after 1, 2 or 3 days in culture.
The rapid advancement in development of biopharmaceuticals and biologics recently has forced the industry to come up better solutions to its problems and issues. Major issues are 1) safety associated with use of animal products, 2) consistency of production associated with variability from lot to lot and 3) cost. Cost of manufacturing by mammalian cell culture is high and hence the high cost of many biologics in the market currently. A small improvement in productivity of a cell line may have a significant savings in terms of cost. Currently there are 21 FDA approved monoclonal antibodies in the market some of which can be life saving to many patients. But high cost may be keeping it from the reach of many. The challenge to the industry is to bring the cost down to be affordable to all.
This article was originally published in BioSpectrum, August 2008
